TECHNIX SPA
EXHIBITOR AT ARAB HEALTH 2025
HALL S1 - STAND H55
HALL S1 - STAND H55
| Address | Via E. Fermi, 45 |
| City | 24050 Grassobbio (BG) |
| Country | Italy |
| Phone | +39-035-3846629 |
| Mobile | +39-392-6387087 |
| supuk@technix.it | |
| Website | www.technix.it |
COMPANY CONTACTS
Federika Supuk: supuk@technix.itCOMPANY KEYWORDS
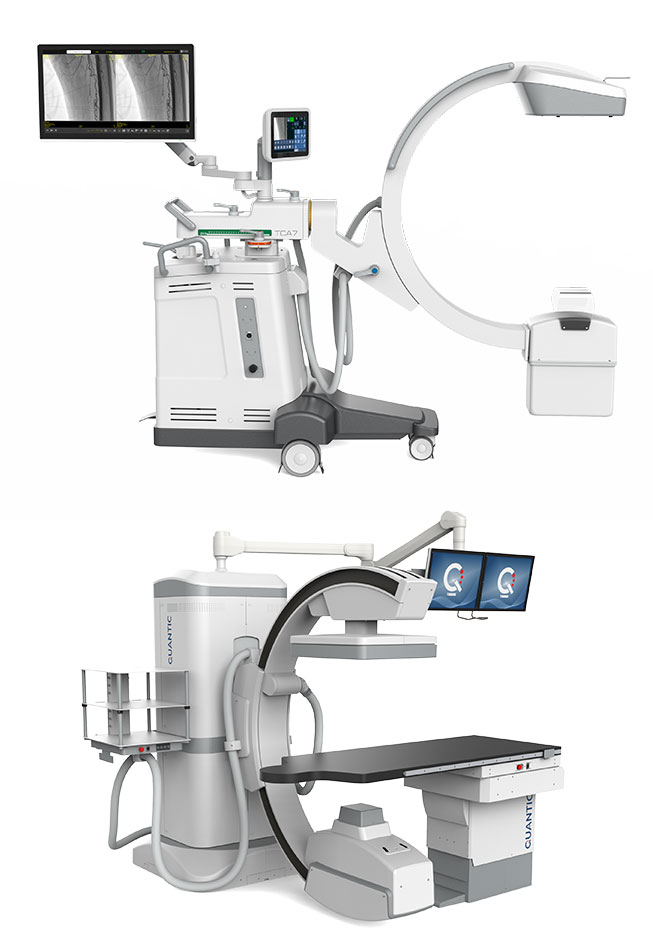
x-ray, radiology, c-arms, mobile x-ray, medical imagingCOMPANY PRODUCT GROUPS
Examination TablesMedical Equipment & Devices
X-Ray Machine
COMPANY PROFILE
Established in 1981, Technix is now Europe's leading manufacturer of X-ray and fluoroscopy devices.The main focus of Technix is the continuous technological innovation aimed at guaranteeing the design, production, distribution and supply of devices in the x-ray field with high technological-qualitative performance with an excellent quality/performance/price ratio. This allows to maintain and consolidate OEM cooperations, but also to make the Technix brand known around the world.
For Technix, assistance means continuous presence and constant support to the customer. Technical Assistance offers the customer the opportunity to have a remote contact or, if necessary, to request direct intervention, for a quick resolution of the problem.
For the sale of its own brand products, Technix avails itself of a network of highly qualified distributors, both for the national market but above all for the international one, as evidenced by the export turnover constantly exceeding 80%. The precious collaboration with our commercial partners has made it possible to reach and exceed the threshold of 25,000 devices installed worldwide.
For Technix, the feedback from its customers and partners who choose it every day is the true certification of quality. One of the main objectives of Technix is the continuous updating of new standards and international regulations, while maintaining customer satisfaction and compliance with the rules at the same time through continuous technological innovation. The constant updating to the most recent regulatory requirements has also allowed Technix to be the first Italian company to obtain, in July 2021, product certification pursuant to Regulation (EU) 2017/745 – MDR. This important milestone confirms Technix’s attention to obtaining and maintaining the highest quality standards in the Medical Devices sector.